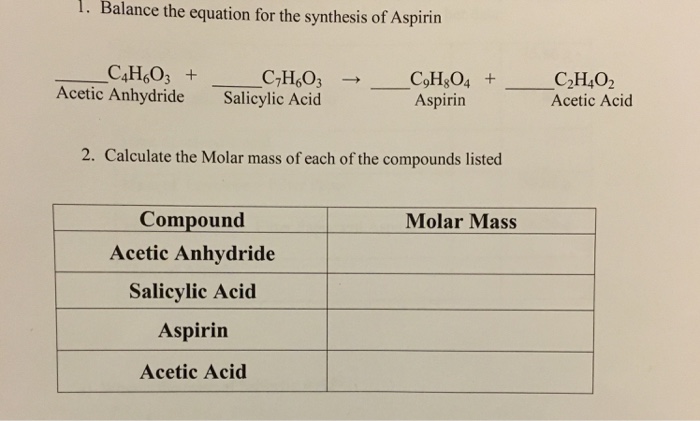
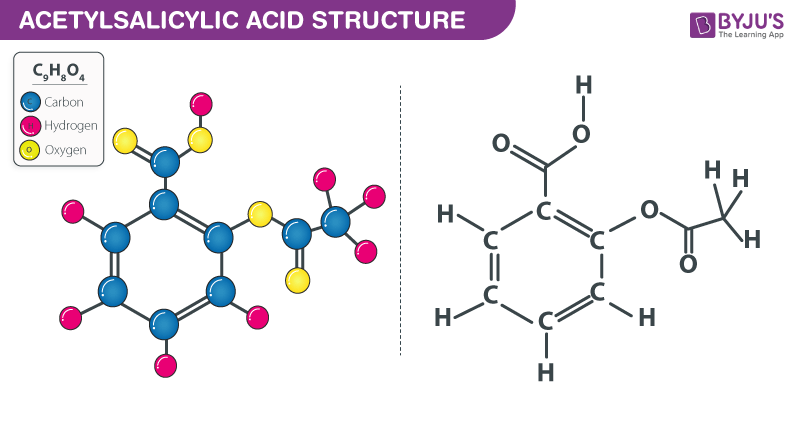
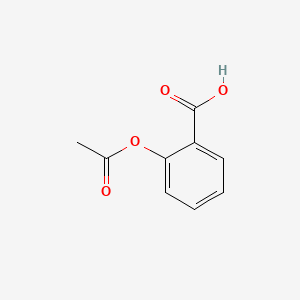
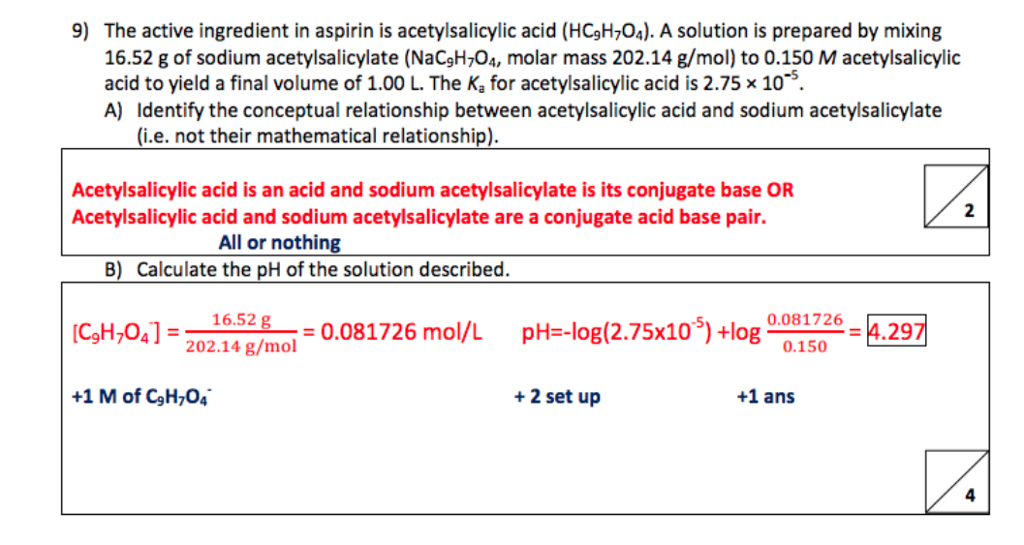
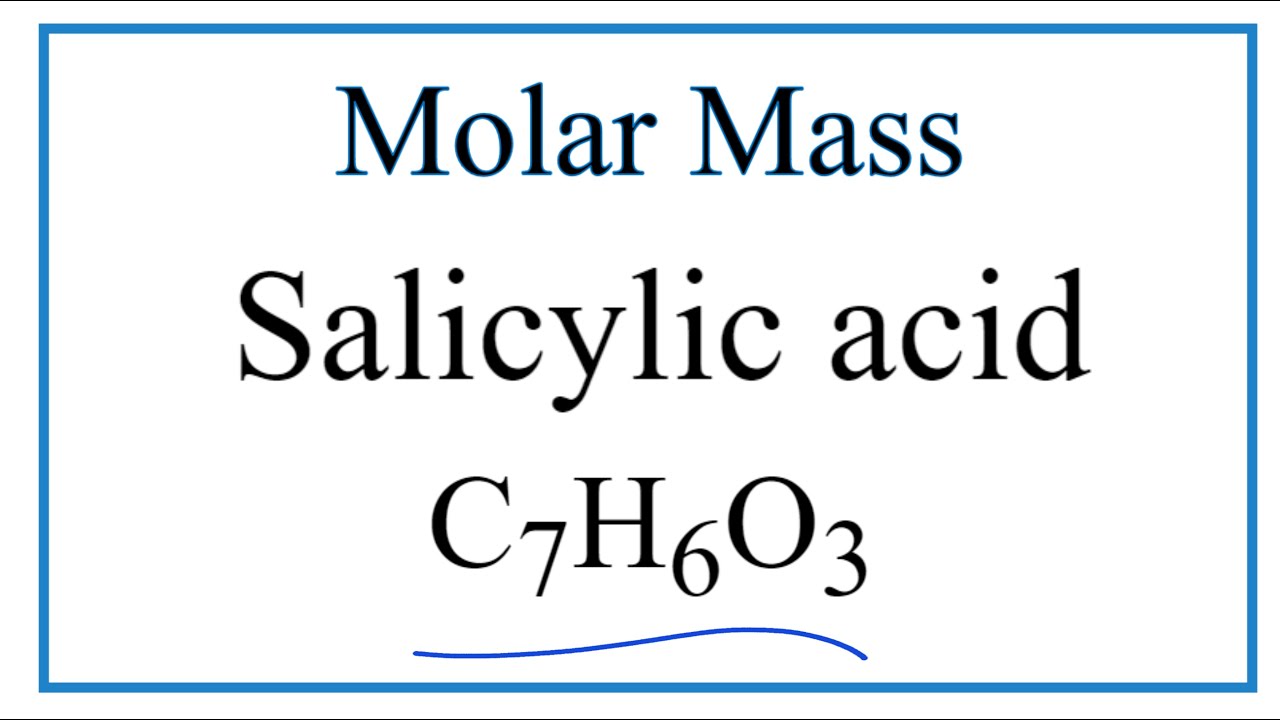SOLVED:The molecular formula of acetylsalicylic acid (aspirin), one of the most commonly used pain relievers, is C9 H8 O4 a. Calculate the molar mass of aspirin. b. A typical aspirin tablet contains

Acetyl salicylic acid (mol. wt. = 180) called aspirin is a pain killer with pKa = 6 . It two tablets each of 0.09 gm mass containing aspirin are dissolved in 100 mL solution. Its pH will be:

SOLVED: Salicylic acid: 2 grams Acetic anhydride: 4 mL Synthesized aspirin: 2.251 g The molecular mass of acetylsalicylic acid is 180.57 g/mol. Based on the number of grams of aspirin that you
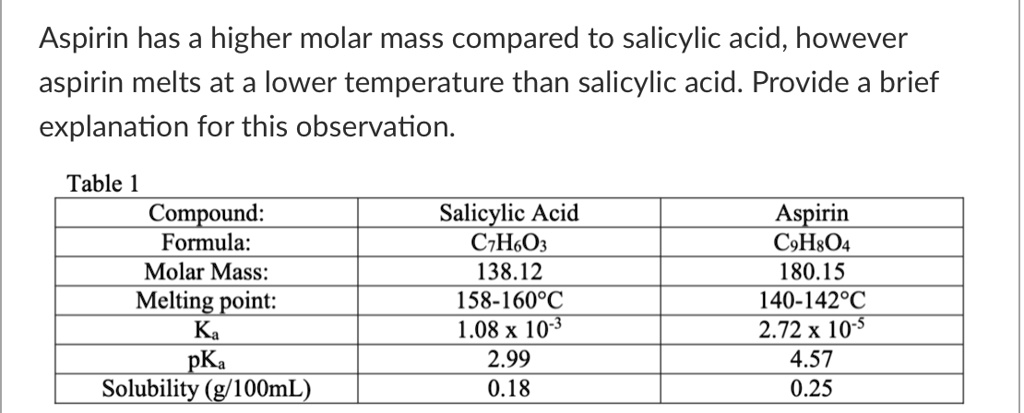
SOLVED: Aspirin has a higher molar mass compared to salicylic acid; however, aspirin melts at a lower temperature than salicylic acid. Table 1: Compound, Formula, Molar Mass, Melting Point, Ka, pKa, Solubility (

Chemical structure and molecular weight (m.w.) of traditional aspirin,... | Download Scientific Diagram

Sketch the molecular structure of acetylsalicylic acid and calculate its molar mass. | Homework.Study.com
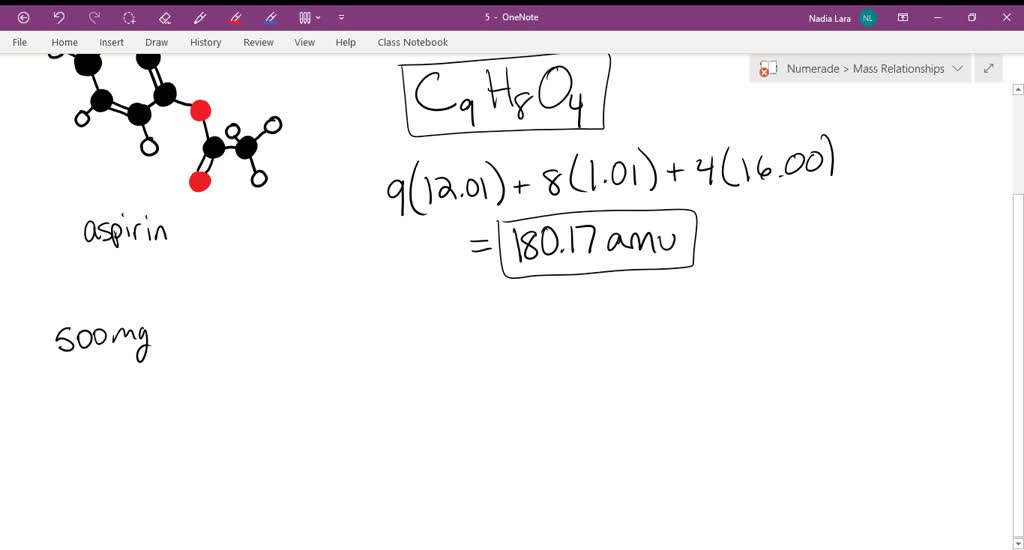
SOLVED:Aspirin can be represented by the adjacent ball-and-stick molecular model. Give the formula for aspirin, and calculate its molecular mass (red =O, gray =C, ivory =H ). How many moles of aspirin

Calculate the mass percentage of aspirin (C9H8O4) in acetonitrile (CH3CN) when 6.5 g of C9H8O4 is dissolved in 450 g of CH3CN .

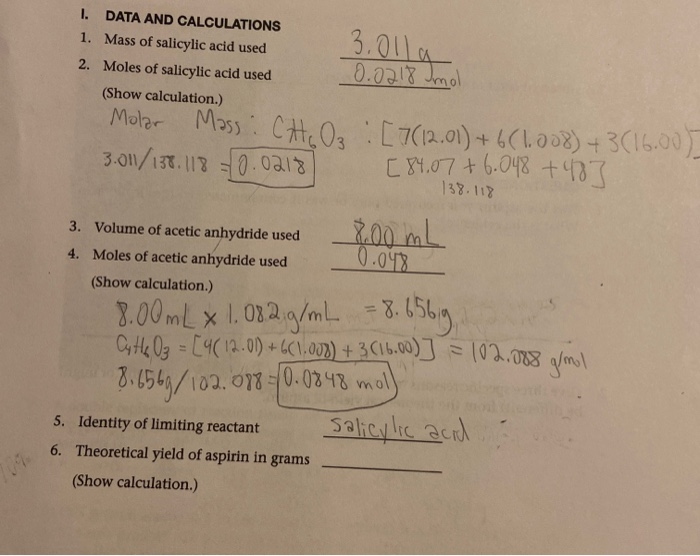
SOLVED: DATA AND CALCULATIONS Mass of salicylic acid used: 3 g Moles of salicylic acid used: 0.2 mol (Show calculation) Molar mass (M) of salicylic acid: C7H6O3 (76.01 g/mol) 3 g /

Calculate the mass percentage of aspirin `(C_(9)H_(8)O_(4))` in acetonitrile `(CHM_(3)CN)` when ... - YouTube